LEVARE
We are delighted to share the exciting news of a strategic partnership between Newsome Park Strategies and Ultimaxx Health, a collaboration set to transform the landscape of pain relief. Together, we are proud to introduce LEVARE, an all-natural, non-narcotic, and non-habit-forming pain reliever designed to revolutionize how we address common ailments.
In an era where the safety profile of traditional over-the-counter (OTC) pain relievers often comes into question, LEVARE stands as a beacon of innovation. Unlike its counterparts such as ibuprofen, aspirin, and acetaminophen, LEVARE offers a holistic approach to pain relief without the associated risks of stomach lining damage or the potential for off-label use complications.
Dr. Leonard Lomax, President and CEO of Ultimaxx Health, and esteemed Senior Medical Advisor for Newsome Park Strategies, spearheads our commitment to “Changing the World Naturally.” With a wealth of experience and expertise, Dr. Lomax and his team have meticulously crafted LEVARE, drawing inspiration from natural elements deeply rooted in Eastern medicine. Each component of LEVARE has undergone extensive research, ensuring its efficacy and safety.



LEVARE®
ULTIMAXX-HEALTH
Out of Stock
$12.95 $15.95
Out of Stock
$34.95 $39.95
Out of Stock
$49.95 $54.95
- Non-Narcotic Pain Relief
- Non-Habit Forming
- Fast. Long Lasting Support
Description
Ready to experience relief? LEVARE® is a revolutionary non-narcotic formula that combines 12 specific natural ingredients (herbs) to provide natural pain relief support without dangerous side effects.
Supplement Facts
Serving size:
600mg/tablet
Proprietary Blend:
- Paullinia Tomentosa
- Chiococca Alba Extract
- Mimosa Pudica Extract
- Phellodendron Amurense Extract
- Lactuca Virosa Extract
- White Willow Bark Extract
- Turmeric Extract
- Harpagophytum Procumbens Extract
- Boswellia Extract
- Naringen (fruit)
- 6-7Dihydroxybergamottin (fruit)
- Yerba Maté (leaves).
OTHER INGEDIENTS:
- Microcrystalline Cellulose
- Dextrose
- Sodium Starch Glycolate
- Magnesium Stearate
- Stearic Acid
- Silica
- Carnuba Wax
Directions:
Take 2 tablets every 4-6 hours as needed for relief of pain. As individual results will vary, dosage may be adjusted to achieve the desired effects (e.g. 3-4 tablets for severe pain). However, we recommend to not exceed 12 pills in any given 24 hour period.
These Statements have not been evaluated by the Food and Drug Administration. This product is not intended to treat, cure or diagnose any disease.
Consult with your physician before starting LEVARE® as a pain relief program if you are currently taking prescription medications. Do not take this product with NSAID’s, aspirin or acetaminophen without consulting with your physician. Do not take this product if you have an allergy to any of its ingredients, to aspirin, are pregnant or nursing. Children and teenagers should not use this product for relief of Chicken Pox or Flu-like symptoms.
LEVARE® Clinical Trial Data
Figure 1: Responder Response to Symptoms Across Pain Scales
FIgure 1 shows patient response to treatment with LEVARE. Responders were asked to rate their level of pain relief after using LEVARE as either poor, okay, good, amazing or revolutionary. 56 (90.4%) of patients rated their response as good, amazing or revolutionary. Significantly, 38 patients rated their response either amazing or revolutionary. Overall response to treatment was similar when patients were assessed based on their initial assessment of pain rated as mild, moderate, or severe. Of all the patients followed, only 1 patient (in the severe pain group) rated their response as poor. However, even in this group of patients with severe pain, 8 of 15 patients rated their pain relief as amazing or revolutionary while the remaining 6 patients rated their relief as good or amazing.Onset of Action ranged from 10 minutes to 1 hour with 76% experiencing relief within 30 minutes and the remaining 24% within the first hour.
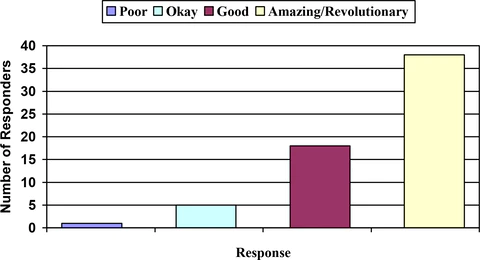
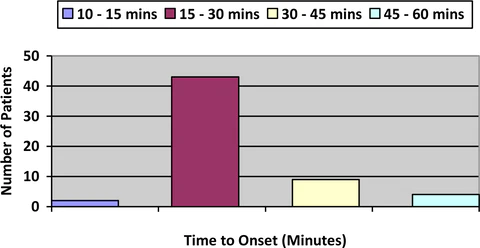
Figure 2: Onset of Analgesic Effect
Figure 2 shows the onset of action of LEVARE. Onset of action was predictable, with most patients, Seventy six (76%) percent achieving pain relief within 30 minutes after dosing. Ninety four (94%) percent of patients achieved relief within 45 minutes and after 1 hour all patients reported having relief of pain.
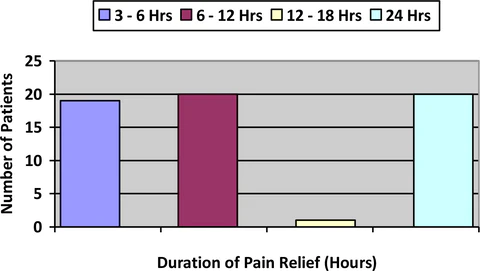
Figure 3: Duration of Pain Relief
Figure 3 summarizes the duration of pain relief achieved with use of LEVARE. Duration of relief ranged from 3hrs to all day; one patient even reported relief of osteoarthritis pain for 4 days. Thirty four (34%) percent experienced at least 6-16 hours of relief and thirty three (33%) percent reported all day relief of symptoms.